., . , OH
реклама
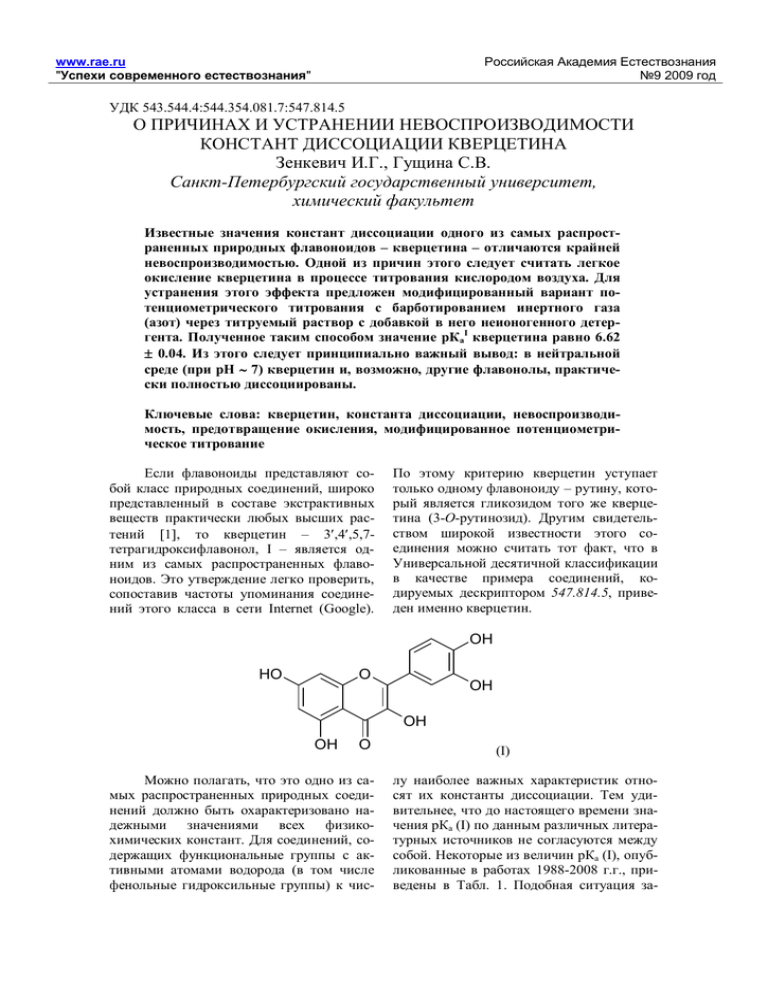
www.rae.ru " " 9 2009 543.544.4:544.354.081.7:547.814.5 ., . , - – – . . ) . 0.04. ( 6.62 I : 7) , , , - . : , , , - , , – – 3 ,4 ,5,7, I – , Internet (Google). 1, . (3- , - , - ). , 547.814.5, . OH O HO OH OH OH O , . , - (I) . , (I) . (I), 1988-2008 ., ( ) - . 1. - www.rae.ru " " 9 2009 1) 2) - . 1. 8.21 7.03 5( ) 6.9 0.6 8.9 7.65 7.59 0.06 8.30 1988 1998 2001 2005 2005 2006 2008 2008 *) I – I II III II III II , II – , III – - . (20-22 100 50% 4.6-4.8) -80 ; pKa , Sigma) ( 25-50 0.5 0 5 - 0.01 - NaOH. - (20) - . 20.0 - , . - (I) 50 10 . 1. , 11 10 9 pH 8 7 6 5 4 0 5 10 15 , . 1. 20 www.rae.ru " " 9 2009 2,4,6- ). (I) ( , ( ). ), ), . , 2 - 3,4- ( (I) , . , « ») 200 , 760 (0.29 .: ( 8.8-9.4 . .) ) 2-4 . . 2 . - ), , - . ( . g, 3 . - (I) – 2 . . , ) , , ). , - ( - . 2, , 5. (I) 1912 ., , 6. , 0.04 - 2007 . (I) pKaI 0.1, 0.2 ( pKaII, ); - 7. (I) ( , - , ( > 7), ) 0.6. 0.7 1.7. - , . , 2. , ( ( ) ) - (pKaI) (pKaII) (Internet) 9.9, 10.0, 9.98, 9.89, 9.98, 9.99, 9.96 9.8, 9.85, 10.35, 10.4, 9.96, 9.9, 9.91, 10.2, 9.8-10.0 11.32, 11.4, 13.0, 12.04, 11.56 9.96 0.04 ) –( 0.1 10.0 0.2 0.6 11.9 0.7 1.7 ) www.rae.ru " " 9 2009 , . , , - , , . (I) ( , 6.62 ( 0.04. - ). - . 1) 6.9 0.6, 8, - 15 . , ( - . , . . 2), 8 . . (I) 6.62 - , ( 8 – 10), ( -80) , 3. - (I) ; , (I) . ... O 6.92, , ): OH O OH O (I) , , II, 2, ( ... ... (I) 6.6, , -1- , - . - , , - (II) (I), 6.6, 9-10 ( 1). , 7.4 ( - ) . II (9.7 . - 0.2), , > 6.6 , . . ( . . 1) - www.rae.ru " " 9 2009 . - (I) , . , (I) > 7, 7. , , , - , . : 1. Flavonoids. Chemistry, Biochemistry and Applications. Eds: O.M.Andersen, K.R.Markham. N.Y.: CRR Taylor & Francis, 2005. 1237 p. 2. Battino R., Rettich T.R., Tominaga T. // J. Phys. Chem. Ref. Data. 1983. V. 12. 2. P. 163178. 3. Vesilind P.A., Morgan S.M. Introduction to Environmental Engineering. 2nd Edn. Boston: Brooks/Cole Publ. Co., 2003. 479 p. 4. Abraham M.H., Whiting G.S., Carr P.W., Onyang H. // J. Chem. Soc. Perkin Trans, 1998. 2. P. 1385-1390. 5. ., ., ., ., . // . . 2008. . 78. 9. . 1449-1456. 6. Brown S.B., Rajananda V., Holroyd J.A., Evans E.G.V. // Biochem. J. 1982. V. 205. P. 239244. 7. Zenkevich I.G., Eshchenko A.Yu., Makarova S.V., Vitenberg A.G., Dobryakov Yu.G., Utsal V.A. // Molecules. 2007. V. 12. 3. P. 654-672. 8. Dubber M.-J. Application of CE, HPLC, LC-MS-MS for the analysis and quality control of Ginkgo Biloba dosage forms. Thesis. Rhodes University, Greece, 2005. . : http://eprints.ru.ac.za/294/01/MJ_Dubber_PhD.pdf ON THE REASONS AND REMOVAL OF IRREPRODUCIBILITY OF DISSOCIATION CONSTANTS OF QUERCETIN Zenkevich I.G., Gushchina S.V. Saint-Petersburg state university, faculty of chemistry Dissociation constants (pKa) of one of the most spread natural flavornoids – quercetin – are characterized by high irreproducibility. The principal reason of that seems to be easy oxidation of this compound by air oxygen during titration. The modified procedure of standard potentiometric titration is proposed to prevent such oxidation. It implies the bubbling of inert gas (niI trogen) through titrated solution contained small additives of non-ionic detergent. Resulted value (6.62 0.04) means that quercetin and, possibly, other flavonols at pH 7 (in neutral solutions) are dissociated in strong extent. Keywords: Quercetin, dissociation constant, irreproducibility, preventing of oxidation, modified potentiometric titration